Addressing Common Challenges in Sourcing the Best Electromyography Equipment for Global Buyers
In the rapidly evolving field of medical technology, sourcing the best Electromyography Equipment has emerged as a critical concern for global buyers. According to a recent report by MarketsandMarkets, the electromyography market is projected to reach USD 1.54 billion by 2025, growing at a compound annual growth rate (CAGR) of 7.6%. This growth underscores the increasing demand for precise diagnostic tools that enhance neurological assessments and patient care.

However, global buyers often face challenges such as varying regulatory standards, differing price points, and the need for advanced technical support. Understanding the benefits of sourcing high-quality Electromyography Equipment can provide buyers with a competitive edge, ensuring they can deliver precise diagnostics while navigating the complexities of international procurement.
Understanding the Importance of Quality in Electromyography Equipment
When sourcing electromyography (EMG) equipment, quality is of utmost importance for global buyers. According to a recent market report by Research and Markets, the global EMG devices market is expected to reach $1.4 billion by 2027, growing at a CAGR of 5.3% from 2020. This growth highlights the rising demand for high-quality EMG equipment, which is crucial for accurate diagnosis and treatment of neuromuscular disorders. Poor quality equipment can lead to incorrect data, resulting in misdiagnosis, ineffective treatment plans, and, ultimately, patient harm.
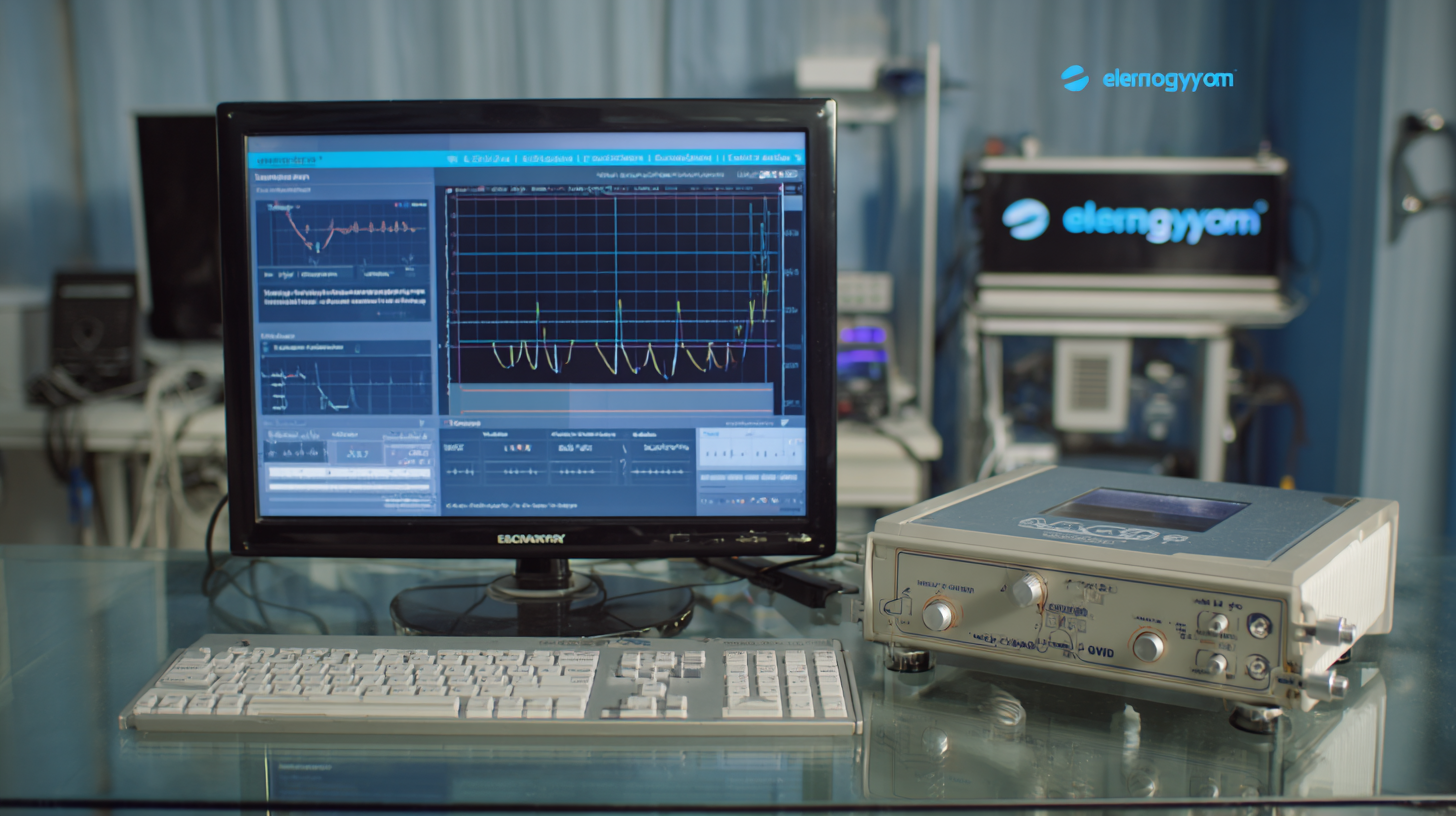
Moreover, a study published in the Journal of Electromyography and Kinesiology indicated that the accuracy of EMG readings is significantly influenced by the quality of the electrodes and amplifiers used. High-quality equipment ensures minimal noise interference and enhances signal clarity, enabling healthcare professionals to make informed decisions. As global buyers navigate through various suppliers, they must prioritize manufacturers who adhere to stringent quality standards and regulatory certifications. Investing in superior EMG equipment not only guarantees better clinical outcomes but also fortifies the trust and confidence of patients in their healthcare providers.
Key Factors to Consider When Sourcing EMG Equipment Globally
When sourcing electromyography (EMG) equipment globally, several key factors must be carefully considered to ensure that buyers make informed decisions in a rapidly evolving market. According to a recent market research study by Custom Market Insights, the global electromyography devices market is projected to reach approximately USD 1,658.9 million by 2032, growing at a compound annual growth rate (CAGR) of around 8%. This robust growth highlights the increasing demand for advanced EMG technologies in clinical and research settings, making it imperative for buyers to stay updated on market trends and technological advancements.
One of the primary challenges faced by global buyers is the wide variation in equipment quality and regulatory standards across different regions. As manufacturers strive to innovate and enhance the capabilities of EMG devices, it becomes crucial to assess not only the technical specifications and features but also the reputation and reliability of the suppliers. Buyers should consider suppliers’ certification, market presence, and customer feedback to mitigate risks associated with sourcing. Aligning these factors with the specific needs of their organizations can lead to better investment decisions in this growing market.
Challenges in Sourcing Electromyography Equipment
This bar chart illustrates various challenges faced by global buyers in sourcing electromyography equipment. The rating scale, from 1 to 10, highlights critical factors such as price, quality, and technical support.
Navigating Regulations and Standards for Electromyography Devices
When sourcing electromyography (EMG) equipment, one of the primary considerations for global buyers is navigating the various regulations and standards that govern these devices. Different countries have specific compliance requirements that manufacturers must meet before their products can be sold, and failing to adhere to these can result in significant delays or even bans on equipment entry. Understanding these regulations not only ensures the safety and effectiveness of the EMG devices but also protects the buyers from potential legal challenges.
Moreover, international standards such as ISO and IEC specifications play a crucial role in the EMG equipment landscape. Buyers must familiarize themselves with these standards to evaluate the quality and reliability of different devices. For instance, equipment that meets ISO 13485 can indicate compliance with quality management system requirements specific to medical devices. As a result, ensuring that the chosen suppliers provide certifications for compliance can streamline the purchasing process and enhance confidence in the equipment's performance and safety features.
Comparative Analysis of Top EMG Equipment Brands and Their Features
When sourcing the best electromyography (EMG) equipment, global buyers face the challenge of selecting from a multitude of brands, each offering unique features tailored to various applications. A comparative analysis reveals significant advancements in EMG technologies, particularly in musculoskeletal research and rehabilitation robotics. For instance, the use of multichannel EMG signals has proven vital in recognizing upper-limb motion patterns during both dynamic and isometric muscle contractions. This capability is crucial for developing more intuitive control systems for exoskeletons and bionic devices.
Recent studies highlight the integration of machine learning with EMG data to enhance application outcomes. The analysis of gait parameters from surface EMG signals demonstrates its effectiveness in assessing mobility and identifying gait disorders, which are vital for rehabilitation strategies. Additionally, leveraging hybrid control mechanisms incorporating EMG-driven intent recognition with neuromuscular electrical stimulation (NMES) is setting new standards in bionic hand control. The continuous improvement and refinement of features among top EMG brands reflect their commitment to addressing the complexities of human motion and enhancing quality of life for individuals with disabilities.
Strategies for Establishing Reliable Supplier Relationships in EMG Sourcing
Establishing reliable supplier relationships is essential for global buyers in the electromyography (EMG) equipment market. One effective strategy is to prioritize communication and transparency. Engaging in regular dialogue with potential suppliers helps clarify expectations and fosters trust, which is critical for long-term partnerships. It’s beneficial to inquire about their manufacturing processes, quality control measures, and service support. A supplier willing to share this information is more likely to be committed to maintaining high standards and reliability.
Another strategy is to conduct thorough research on suppliers' reputations and track records. This includes reading reviews, seeking feedback from other buyers, and examining certifications and compliance with industry standards. Participating in trade shows or industry events can provide opportunities to build connections and assess the commitment of suppliers firsthand. By investing time in due diligence, buyers can identify partners who not only meet their technical needs but also align with their values of quality and customer service. Building these relationships is foundational to successful EMG equipment sourcing in a competitive global market.
